��������������ѧԺʩ˼���Ŷ��ں�LiI��������ˮLi-O2������ܼ������ӷֻ���ӳ��ԭ������ý�չ�������й����гɾ��ԡ�The Origin of Solvent Deprotonation in LiI-added Aprotic Electrolytes for Li-O2Batteries��Ϊ��䷢�ڻ�ѧ����ѧ���ڿ����¹����û�ѧ����Angewandte Chemie International Edition������JCR���п�Ժһ��Top�����������������ĵ�һ����Ϊ��ʿ������ƽ����Ϊ�廪��ѧ��ʿ���������±�GGΪ��һ���ߵ�Ԫ������ʩ˼����ںͽ���ʦ����ѧ�����츱����Ϊ��ͬͨѶ�������������������̺����Ŵ�ѧ������ڡ�����ʦ����ѧ���������ں��Ͼ���ҵ��ѧ����Խ�����������

������������ԭý��������ǽ������������س��߹���λ�������ѹ滮֮һ��������LiI��Ϊһ�ָ�Ч�ͳɱ���������ԭý������������������Li-O2��������ÿ�����������Ȼ����������LiI��Li-O2���ϵͳ���������ܼ�����(��дΪHA)�����ϳ��������ӷֻ���ӳ�����������������м���������LiOH������������LiI�������Ƶ�LiBr������ԭý����ȴ���������ƾ����������ź�����LiBr����Ч��Զ����LiI�������������������ʾLiI/LiBr������ԭý����֮��IJ���ǽ��LiIϵͳ����ӳ���������Li-O2�������Ч�ܺ�ѭ�����ܵĹؼ���������
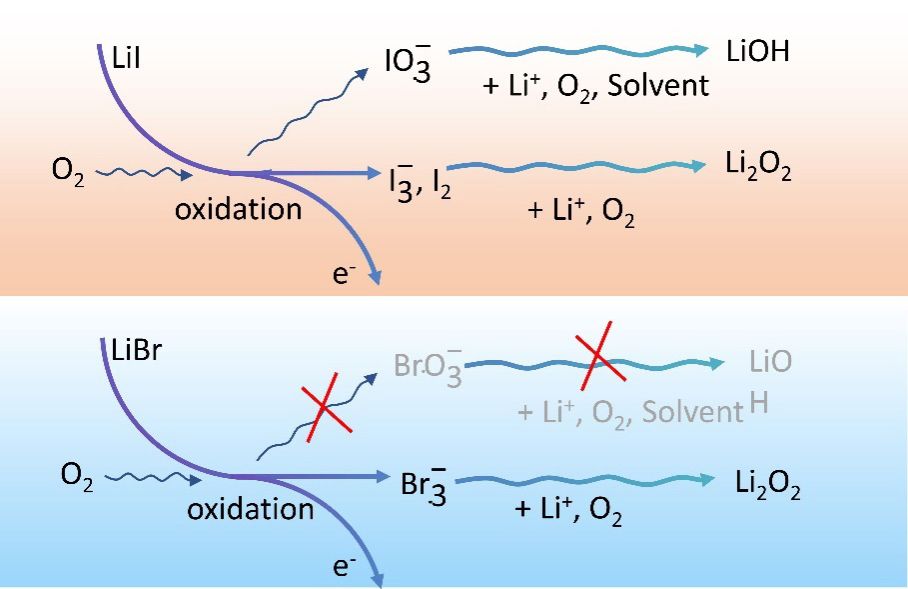
������������������߽������Ԥ���볢����֤������ѡȡ�Ա����е��ַ�������ϵͳ�Ե�������Li-O2�����LiI/LiBr���ܼ���������ɵĻ��ξ��������������з��ֺ�LiI���ϵͳ���������ܼ����������ӷֻ���ӳ��Ҫ��LiI���˸���ƷLiIO3������������LiBrϵͳ����������˹�����ǿ���˸���Ʒ���������÷��ֶ�����ܼ��������������ƶ���Ч�ͳɱ�LiI������Li-O2����е���ʵ�����г�Ҫ������������
�ù����õ����ȳ����з����㡢������Ȼ��ѧ�����������������������
�������ӣ�https://onlinelibrary.wiley.com/doi/10.1002/anie.202217354








